Palladia Medication For Cats

Usually this drug is considered when we have 1 significant disease and knowing this carries a poor prognosis 2 the lack of standardized therapy 3 and palladia is fairly well tolerated in cats.
Palladia medication for cats. A group decrease in feed consumption was apparent in the 1 5x group compared to placebo with the largest decrease in means occurring at day 35. At first it was only open to pet oncologists cancer specialists but now open to use by veterinarians in general. However it may be an appropriate treatment option for some cats. Carcinomas such as squamous cell carcinoma.
This was described in the dog cancer survival guide by it s pre market name su11654. It is expected to be available next year. Palladia was the first antineogenesis and antiproliferative cancer treatment approved by the fda for use with canines. Administer an initial dosage of 3 25 mg kg 1 48 mg lb of body weight every other day.
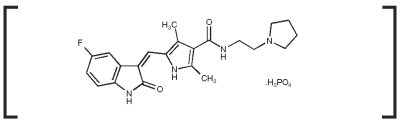
The empirical formula is c 22 h 25 fn 4 o 2 h 3 o 4 p and the molecular weight is 494 46. It is now being used more and more for canine mast tumors tumors derived from so called mast cells in dogs and now even in cats. Dose reductions of 0 5 mg kg every other day and dose interruptions skipping your dose for up to 2 weeks may be utilized if needed to manage adverse reaction at the discretion of your veterinarian. Palladia the first drug officially approved for use in treating dog cancer has arrived.
Mast cell tumours. At the ohio state university palladia is used as a first line treatment for dogs with mcts that exhibit a mutation in a gene called kit. Palladia a multi kinase inhibitor targeting several receptor tyrosine kinases rtk is the phosphate salt of toceranib. However the cons are that it has the potential to cause a variety of side effects such as gastrointestinal side effects hypertension proteinuria neutropenia hepatopathy shifting lameness pancreatitis and cardiotoxicity.
It can also be considered in cats with other cancers such as gastrointestinal stromal tumours gists and pancreatic cancer. Dose reductions of 0 5 mg kg to a minimum dose of 2 2 mg kg 1 0 mg lb every other day and dose interruptions cessation of palladia for up to two weeks may be utilized if needed to manage adverse reactions see table 2as well as warningsand. Palladia can sometimes be safely combined with other anti cancer therapies such as metronomic chemotherapy radiation therapy and some conventional chemotherapy drugs. Palladia is currently licensed by the fda for treatment of canine mast cell tumors mct.
Palladia made by pfizer has been approved by the fda. Palladia is an attractive treatment option for many owners because they can administer the medication orally to their pets at home three times a week. Benefit of palladia in cats with cancer.