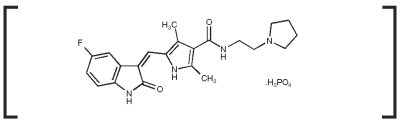
Palladia Dosage For Cats

In cats approximately 20 to 70 experience a side effect from palladia that is usually mild and temporary gastrointestinal signs predominately loss of appetite or bone marrow damage drop in the.
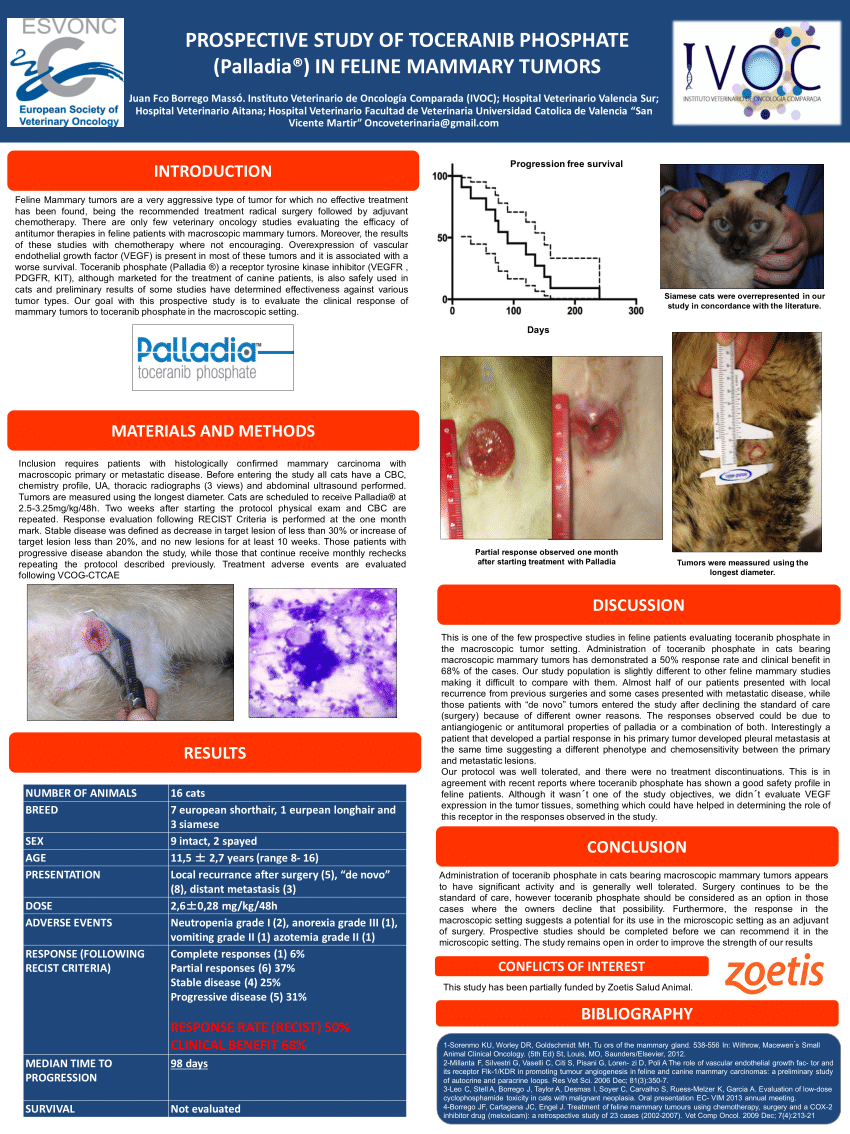
Palladia dosage for cats. Administer an initial dosage of 3 25 mg kg 1 48 mg lb of body weight every other day. All dogs received at least 1 dose of palladia. B investigators assigned severity grade of 1 2 3 or 4 1 least severe. Dose reductions of 0 5 mg kg every other day and dose interruptions skipping your dose for up to 2 weeks may be utilized if needed to manage adverse reaction at the discretion of your veterinarian.
At the ohio state university palladia is used as a first line treatment for dogs with mcts that exhibit a mutation in a gene called kit. Some dogs require dose adjustments and more frequent visits in the first month to try to get the palladia dose right for the individual dog. It is now being used more and more for canine mast tumors tumors derived from so called mast cells in dogs and now even in cats. However it may be an appropriate treatment option for some cats.
Palladia initial dosage for dogs weight dosage. 4 most severe. 4 most severe. Adjust dose based on approximately weekly veterinary assessments for the.
Administer 15 mg orally every other day. Benefit of palladia in cats with cancer. Administer 20 mg orally every other day. Some dogs require dose adjustments and more frequent visits in the first month to try to get the palladia dosage right for the individual dog.
At first it was only open to pet oncologists cancer. Dose reductions of 0 5 mg kg to a minimum dose of 2 2 mg kg 1 0 mg lb every other day and dose interruptions cessation of palladia for up to two weeks may be utilized if needed to manage adverse reactions see table 2 as well as warnings and precautions. C grading of laboratory abnormalities was based on the national cancer institute s common toxicity criteria guideline adapted for canines 1 least severe. Administer 30 mg orally every other day.
Dose reductions of 0 5 mg kg to a minimum dose of 2 2 mg kg 1 0 mg lb every other day and dose interruptions cessation of palladia for up to two weeks may be utilized if needed to manage adverse reactions. Palladia was the first antineogenesis and antiproliferative cancer treatment approved by the fda for use with canines. Administer 35 mg orally every other day. In cats approximately 20 to 70 experience a side effect from palladia that is usually mild and temporary gastrointestinal signs predominately anorexia or bone marrow suppression neutropenia.
Palladia tablets were administered orally to 20 male and 20 female adult dogs approximately 2 years of age at doses of 0 mg kg placebo 12 dogs 2 mg kg 0 5x 8 dogs 4 mg kg 1x 12 dogs or 6 mg kg 1 5x 8 dogs once every other day for 13 consecutive weeks without dose interruption.